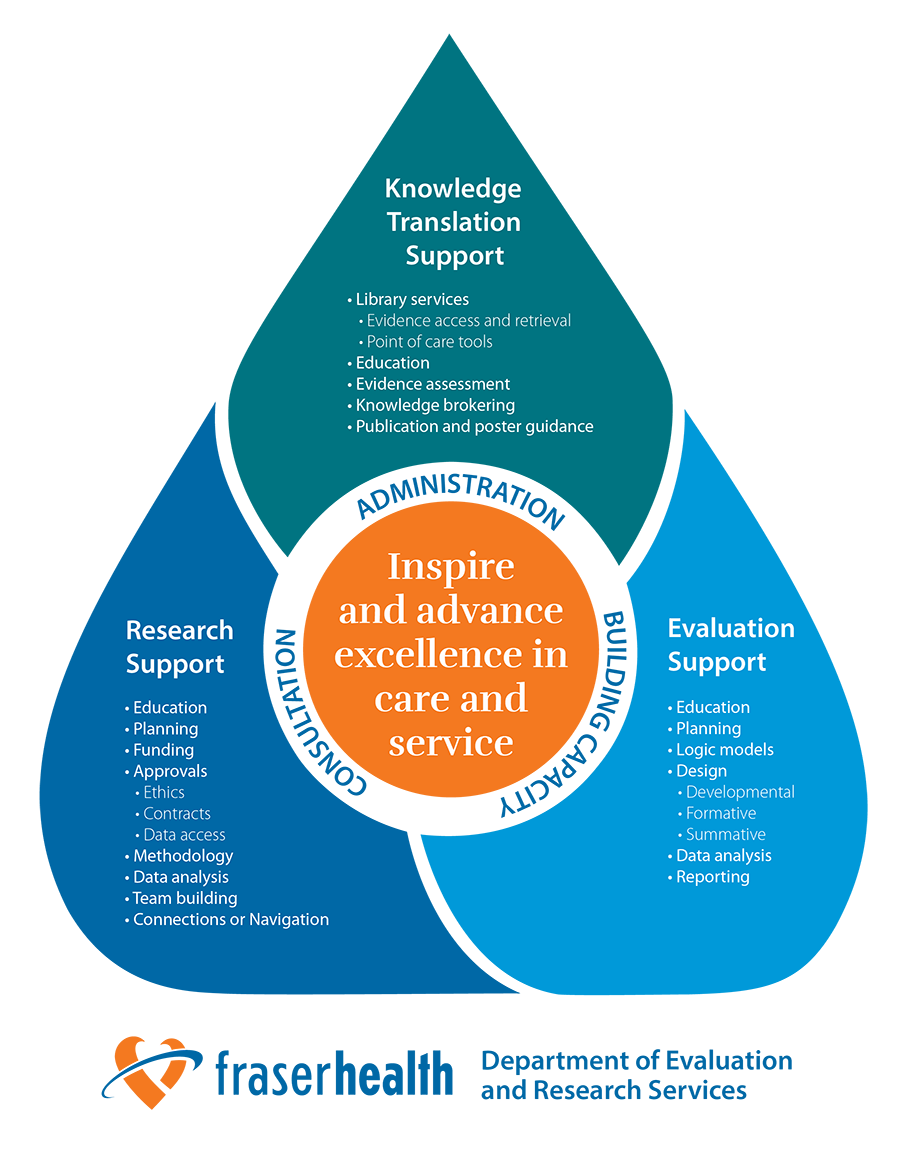
We offer a variety of services and support to facilitate your research, evaluation and quality improvement projects.
The Department of Evaluation and Research Services aims to enable Fraser Health to be a leader in developing research that maximizes the well-being of the patients, clients and residents that we serve. We promote excellence in every care experience by integrating research into practice. We do this by providing capacity building services that enable researchers to use knowledge for the benefit of residents across the region.
We support research in public and population health, clinical intervention, health services/systems, health care technology and innovation, knowledge transfer and implementation, as well as program evaluation and quality improvement studies.
Learn about the differences between research, quality improvement and evaluation.
Download our Department of Evaluation and Research Services fact sheet.
What services do you provide?
-
Methodology unit
The methodology unit provides specialized consultation services for research, evaluation/economic analysis, and knowledge transfer and exchange.
Research
- Priority setting for development of a research program
- Research project/protocol development including ensuring research question is congruent with study design for both quantitative and qualitative research
- Statistical analysis services including development of statistical plan, sample size calculation, database design, data analysis (excludes data entry), and interpretation
- Support with study dissemination (poster/abstract/manuscript review)
- Project management planning and team development
- Research protocol development including ensuring research question and type of research meet funding criteria
- Critical review and submission of application to funding agencies, including proposal and budget development
- Pre- and post-award grant administration
- Financial administration: research accounts set up for research awards held by Fraser Health researchers
- Affiliation agreement approval for non-Fraser Health academic researchers
Evaluation
- Evaluability assessment
- Program evaluation planning including development of the evaluation design, methods, statistical/qualitative analysis plan, analysis, and interpretation
- Logic model development
- Evaluation implementation planning
- Report writing and dissemination
- Economic analyses, including cost effectiveness analyses
- Health technology assessment
-
Knowledge transfer and exchange
Literature searches and publication retrieval including methodology for:
- Systematic reviews (Cochrane review methodology)
- Scoping reviews
-
Research coordination
- Priority setting for development of a research program
- Facilitate development of new research ideas from concept to protocol
- Develop strategies to identify funding opportunities and support, including collaborations
- Research start-up: document management, standard operating procedures, study budget preparation, study staffing, clinical trial agreement process oversight, creation of case report forms and other required source documents
- Facilitate knowledge transfer of research results
- Oversee implementation of the ‘consent to contact’ process
- Negotiate clinical trial agreements with industry and academic partners
- Identify opportunities and promote partnerships and alliances to support business development in research ventures
- Act as a research ‘navigator’ to match employees and physicians with right resources to support research development, including protocol development, regulatory management, Health Canada submissions, research start-up and maintenance
- Oversee implementation of the ‘consent to contact’ process in consultation with site partners
-
Education and communications
- Workshops for research and evaluation skills training
- Knowledge dissemination activities, such as Research Week and Researchers’ Cafes
- Knowledge products, such as publications by posting to the Fraser Health Research Study Database
- Implementation of knowledge dissemination for research
- Coordination of all communication activities
-
Library services
- Knowledge dissemination via library liaison representatives for clinical programs
- Distribution of current publications to clinical programs
- Workshops for library skills training and document management
-
Research ethics review
- Consultation for ethics submissions to the Fraser Health Research Ethics Board
- Compliance with annual review requirements
- Research ethics workshops
- Research Quality Improvement Program for mock Health Canada inspections
- Clinical trial registration
-
Administration
- Strategic planning, policy development and administration, including collaborations with health authorities, universities, research institutes and funders
- Liaison with departments that provide research-related services, such as lab, pharmacy, imaging and health records
- Clinical trial agreement approval for industry/academic sponsored research
- Oversight for affiliation agreement process for non-Fraser Health academic researchers
- Authorization of final approval for all research studies
- Executive briefings for high impact research studies
- Financial administration oversight for grant and industry funded research
- Maintenance of all research study documentation
-
Medical research and development
- Strategic planning for the development of medical research in Fraser Health
- Coordination of medical research development in collaboration with universities, research institutes and health authorities
- Promotion of research opportunities and research support for Fraser Health physician investigators
- Facilitation of research proposal development for physician led research
Strategic plan
The 2009-2014 Strategic Plan outlines the following:
- Strategic planning process
- Key messages
- Proposed research agenda
- Strategic goals
- Key success factors
Annual report
Our 'Year in Review' annual reports highlight key research success stories in addition to providing a snapshot of our department’s activity.
-
pdf207.41 KB
-
pdf149.62 KB
-
pdf112.48 KB
-
pdf116.86 KB
-
pdf118.35 KB
-
pdf91.77 KB
Research Ethics Board annual report
Quality framework
The integrated research quality framework applies to the conduct of research and promotes high quality research for the benefit of its patients, clients and residents.
-
pdf380.34 KB