Learn about the steps to conduct research. From generating your research idea to disseminating your findings.
Updated December 21, 2022
The Department of Evaluation and Research Services (DERS) supports researchers in developing and using research to maximize the well-being of patients, clients and residents.
How do I create a research plan?
The following are key steps in the research development process:
-
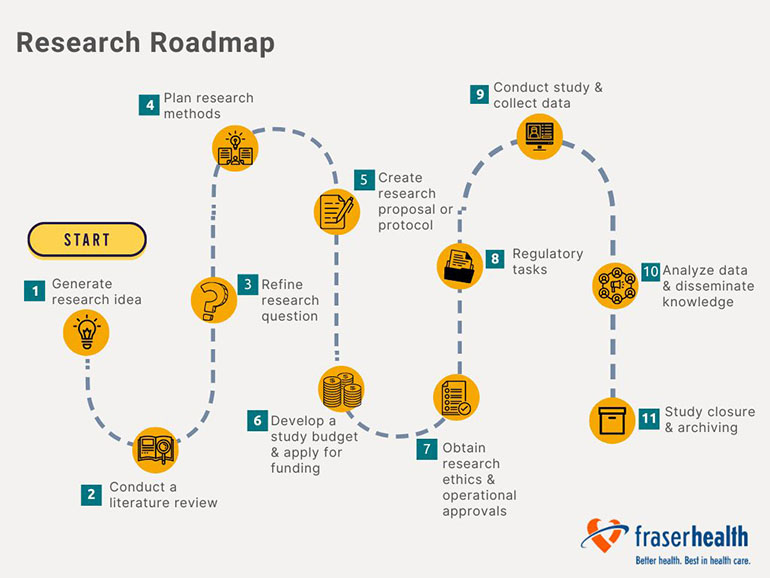
Step one: Generate research idea
You have an exciting research question that you want to explore.
Learn about the differences between research, quality improvement and evaluation.
Once your initial research idea has been defined, it is necessary to think about research intent and previous research.
- What do you hope to accomplish?
- Has this research been done before?
Discuss the question with colleagues and find collaborators. Go to Fraser Health Writes for a database for published material created by Fraser Health staff and physicians as well as a directory of Fraser Health researchers. Go to Research Study Database to access studies that have been conducted in Fraser Health since 2005.
-
Step two: Conduct a literature review
Conducting literature reviews take time, but this is a very important step in the research process. Contact our library services department to begin the process.
- Decide on the scope of the review (specific or narrow?).
- Review books, journal articles and other peer-reviewed sources such as registries of evidence-based research literature.
- Review the references section of relevant articles to aid in the search for related articles.
- Access the grey literature.
- Keep track of the process used in the searches.
- Consider the quality of evidence being searched (use critical appraisal tools for evaluating quality of evidence).
- Identify gaps in existing knowledge.
-
Step three: Refine research question
Now that you have exhausted the literature, how has your original research idea changed?
- State the research question.
- Include strong justification(s) to the proposed research question. What are you adding to existing knowledge?
- Is your question feasible? Consider your timelines, team member expertise, and settings.
Your research question should guide the study design, method and analytical plan.
-
Step four: Plan research methods
Provide a description of the proposed study, significance and how the study is being conducted to answer your research question.
Include patients as partners during the planning of the research. The Fraser Centre provides services to support patient-oriented research.
Consider the following:
- What is your research objective?
- Choose a methodology (qualitative vs. quantitative research).
- Include research design (observational or experimental) or theoretical framework (grounded theory).
- Identify the sampling method (probability vs. non-probability; purposeful).
- Decide on the study population and study sample (inclusion/exclusion criteria).
- Identify relevant measures/tools to be used in the study.
- Consider the reliability and validity of chosen tools (time to complete, costs).
- Consider the data collection method.
- What kind of data do you plan to collect?
- What collection method will you use? Questionnaire? Previously collected data? Test results? Medical records data?
- Consider using an electronic data capture (EDC) platforms to collect the data.
- Consider sample size (sample size calculations/justification).
- Develop a statistical analyses plan.
- Consider the variables you wish to measure, and review the statistical test you wish to use in order to understand if it is appropriate for what you wish to do.
Contact our research and implementation scientist and research development specialist for supports on study method development.
-
Step five: Create research proposal or research protocol
Create a research proposal that will enable people who are not involved in the study to understand exactly what you plan to do. A proposal will be helpful when applying for grant funding.
Your proposal should include:
- Introduction
- Literature review
- Purpose
- Expected benefits
- Hypotheses
- Objectives
- Research design
- Data collection techniques
- Statistical analysis plan
- Develop knowledge dissemination plan
-
Step six: Develop a study budget and apply for funding
Create a budget to cover all expenses related to the conduct of the study, which may include both direct costs and indirect costs.
Visit budgeting for additional information.
Visit funding opportunities and grant application process to get additional information about funding opportunities and research grant application process, respectively.
-
Step seven: Obtain research ethics and operational approvals
Prior to conducting your study, you need to obtain Fraser Health Institutional Approval, via a Letter of Authorization to Conduct Research (LOA), which requires both ethics approval and operational approval (Department Agreement(s) to Provide Research Related Services, Data Access Agreement, Contracts, etc.). Go to Research approval at Fraser Health for more detailed information.
-
Step eight: Regulatory tasks
Register your research in a public registry, such as ClinicalTrials.gov.
If your study is a regulated clinical trial, you need to submit Health Canada Clinical Trial Applications (CTAs), in addition to the ethics and operational approval. Health Canada also requires investigators to create and maintain the essential documents (e.g. training documentation, standard operating procedure), which you can do in either a physical binder or an electronic regulatory management system, such as Clinical Trial Management System – eDocs.
For non-regulated clinical trials, it is recommended to also create and maintain the essential documents to comply with Good Clinical Practice (GCP) guidelines.
-
Step nine: Conduct study and collect data
Prior to collecting data:
- Develop data dictionary (or code book)
- Develop data files (spreadsheet) or use an electronic data capture (i.e. REDCap) to enter the data, considering study design and analyses plan
You can request access to REDCap for free through Population Data BC / Michael Smith Health Research BC for a secure data storage and management. REDCap is a secure web platform for building and managing online databases and surveys.
Recruit and enroll patients to the study. Resources for participant recruitment:
While collecting data:
- Conduct regular quality checks to identify data entry errors
- Use logic check to clean the data and to identify data errors
- Exercise care in the collecting and coding of data
-
Step ten: Analyze data and disseminate knowledge
Once the study is complete, it is time to analyze the data, write up your findings and formulate conclusions.
- Was your research question answered?
- What has been learned? What are the study implications?
- What findings were significant? Not significant?
- Why do you think this was the case?
- How are your results related to other studies from your literature review (add, support or contradict)?
- What were the limitations of your study?
- What about ideas or recommendations for future research?
Contact The Fraser Centre for knowledge translation support and for media coverage request.
Update results on ClinicalTrials.gov, if applicable.
-
Step eleven: Study closure and archiving
Retain documents per the applicable policies and regulations.
